Abstract
Background: Sickle cell disease (SCD) induces a chronic prothrombotic state, with a cumulative incidence of venous thromboembolism (VTE) reported to be 11% by age 40. Central venous access devices (CVAD) are commonly used for chronic transfusions and iron chelation in this patient population.The presence of a CVAD is an additional risk factor for venous thromboembolism (VTE), with a catheter related thrombosis rate of 24%. Despite this high risk of VTE, the role of thromboprophylaxis in this setting is uncertain due to a lack of high quality data.
Methods: A survey was administered in March 2021 to physicians caring for adult sickle cell disease patients via the Canadian Haemoglobinopathy Association (CanHaem), covering nine SCD comprehensive care centers in Canada. One reminder email was distributed after 3 weeks to encourage participation. Questions were directed at characterizing the practice size, number of patients with CVADs, and the role of thromboprophylaxis for CVADs. Physicians were also surveyed about their willingness to enroll their SCD patients with CVADs in a randomized trial of thromboprophylaxis versus placebo. Items were generated and selected based on face and content validity. Results are reported in medians and percentages, where applicable.
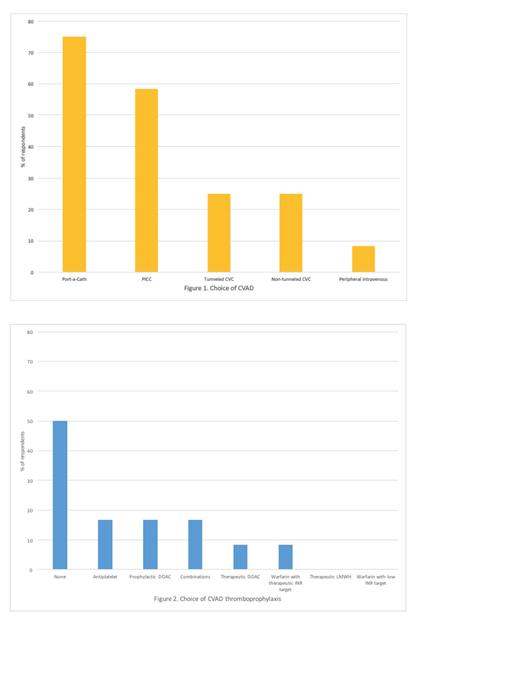
Results: Responses were collected from 14 physicians who care for a median of 100 (IQR 185) adult sickle cell disease patients in practices across Canada. Physicians reported approximately 5% of their patients currently require a CVAD, and physicians estimated no CVAD patients are lost to follow up. Respondents use a variety of CVADs, including port-a-caths (75%), followed by PICC lines (58%), tunneled (25%) and non-tunneled CVCs (25%) (Figure 1). Duration of venous access was reported to be <1 month (17%), 1-3 months (8%), 3-6 months (0%), 6-12 months (8%), and >12 months (67%). Fifty percent of respondents indicated they do not use thromboprophylaxis for CVADs. Responses varied with respect to choice and dose of antiplatelet or anticoagulant in cases where thromboprophylaxis is used (Figure 2). Forty-two percent of physicians indicated they were not very confident or not at all confident in choice of prophylaxis. Past history of VTE was the most cited factor influencing the choice to use thromboprophylaxis. Physicians were generally in favour of enrolling patients in an RCT using thromboprophylaxis for CVADs. The exception was that 69% answered "No" when asked about enrolling patients with a prior history of VTE who are not currently on anticoagulation. One-hundred percent of physicians agreed that an RCT would improve their confidence in decision-making around thromboprophylaxis in their patients with CVADs.
Conclusions: While there is evidence for an increased risk of VTE for SCD patients with CVADs, our results suggest there remains clinical equipoise with respect to the use of thromboprophylaxis. Thromboprophylaxis options were variable when physicians chose to use them, as there is no evidence to support specific antithrombotic regimens. All physicians surveyed are supportive of an RCT to clarify this management approach, and many would enroll their patients. As a result of this survey, a Canadian multicenter pilot RCT addressing this question is currently underway.
Forte: Pfizer: Research Funding; Canadian Hematology Society: Research Funding; Novartis: Honoraria. Verhovsek: Vertex: Consultancy. Kuo: Alexion: Consultancy, Honoraria; Celgene: Consultancy; Bluebird Bio: Consultancy; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria; Apellis: Consultancy.
This survey explored the use of LMWH, direct oral anticoagulants, warfarin and ASA for prophylaxis among patients with sickle cell disease using a central venous access device.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal